|
All our vaccines undergo very strict
toxicology studies before they can be used in human clinical
trials. To date, various combinations and doses of the DNA,
MVA and FP9 vaccines have been used safely in a number of
human trials involving small numbers of volunteers, both
in Oxford, UK and in The Gambia, West Africa with very encouraging
results.
|
 A volunteer receiving a vaccination
A volunteer receiving a vaccination |
|
| Trial
number |
Vaccine
Regime |
No.
of Vaccines |
No.
challenged |
| VAC 01 |
DNA IM vs DNA gene gun +/-
MVA boost |
12 |
|
| VAC 02 |
MVA |
6 |
|
| VAC 03 |
DDDM, DMM, DDD, MMM |
15 |
12 |
| VAC 04 |
Challenge study for VAC
03 |
|
5 |
| VAC 05 |
DDMM vs GGMM |
9 |
9 |
| VAC 06 |
DDMM vs MMM in The Gambia |
18 |
|
| VAC 07 |
Challenge study for VAC
05 |
|
5 |
| VAC 08 |
MVA boosting of challenge
controls |
4 |
|
| VAC 09 |
MVA - safety study of higher
MVA dose (1.5 x 108 pfu) |
6 |
|
| VAC 10 |
DDDMM - higher dose DNA
(2mg) + higher dose MVA, longer interval |
9 |
9 |
| VAC 11 |
MVA in children 1 to 5 in
The Gambia |
20 (+19controls) |
|
| VAC 12 |
FP9 safety study and FFM
i.e. FP9 twice followed by high dose MVA boost |
12 |
5 |
| VAC 13 |
Challenge study for VAC
10 and VAC 12 |
|
10 |
| VAC 14 |
FFM, DDM, DDDM (all at higher
doses) in The Gambia |
29 |
|
| VAC 15 |
FFM vs MMM (all at higher
doses) |
17 |
16 |
| VAC 16 |
Challenge study for VAC
15 |
|
5 |
| VAC 17 |
DDFM, DDMF, FM, MF |
17 |
17 |
| VAC 18 |
RTS,S and MVA-CSO study:
RRMcs, McsRR |
24 |
(16) |
| VAC 20 |
Double-Blind Randomised
Efficacy Trial of DDM with Rabies Controls |
372 (1:1) |
? |
| Vac 21 |
DDM-ME TRAP v. DDM-CS |
16 |
|
| Vac 21.2 |
Challenge study for Vac 21 |
|
22 |
| Vac 22.1 |
ICC-1132 in Seppic ISA 720 |
11 |
|
| Vac 22.2 |
Challenge study for Vac 22 |
|
17 |
| Vac 23 |
FFM-CS and FFM-CS/ME-TRAP |
32 |
21 |
| Vac 24 |
FP9 ME-TRAP and MVA ME-TRAP in children 1 to 6 in Kenya |
22 |
|
| Vac 25 |
FP9 CSO and MVA CSO in Kenya |
20 |
|
| Vac 26 |
FP9 CSO and MVA CSO in The Gambia |
32 |
|
| Vac 28 |
FP9 CS and MVA CS |
21 |
|
| Vac 29 |
PCR diagnosis to evaluate pre-erythrocytic malaria vaccines |
102 |
|
| Legend |
| D |
DNA vaccine (intramuscular) |
| M |
MVA vaccine (intradermal) |
| F |
FP9 vaccine (intradermal) |
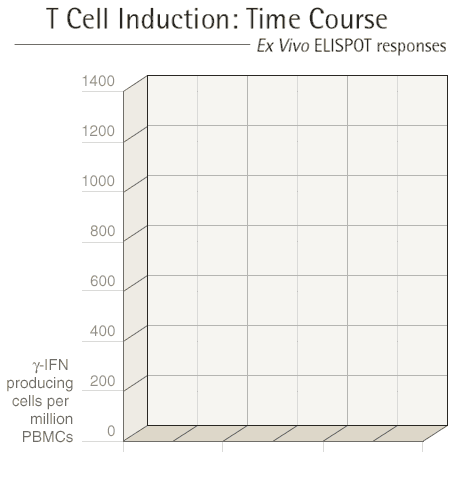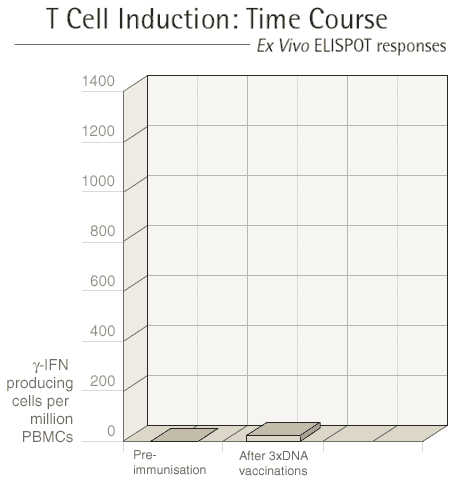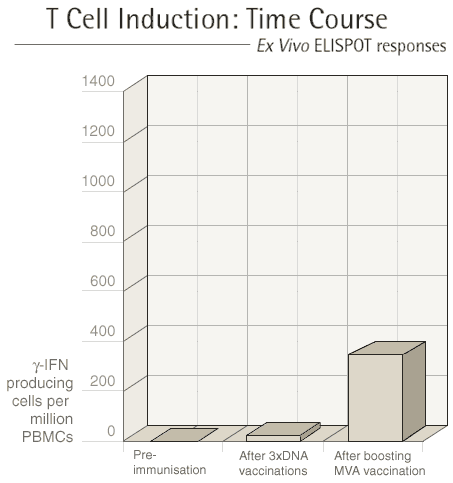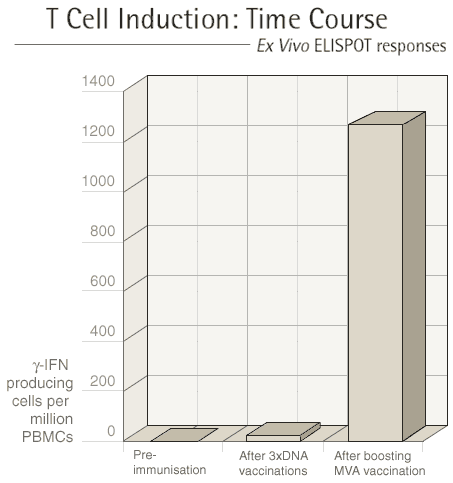
Each vaccination regime involves at least two different
types of vaccine and these are given at three to four week intervals.
After each vaccination, we see our volunteers one week later to
measure the immune response that they have made to the vaccine.
The first type of vaccine given "primes" the immune
system and the second type of vaccine "boosts" the immune
response.
|
After vaccination, it is important to
see whether the vaccines are able to protect people against
malaria. In order to do this we infect our volunteers with
a fully drug-sensitive strain of malaria using a very safe
and well-established procedure at Imperial College in London.
In addition to vaccinated volunteers, we also have a number
of unvaccinated controls take part in the challenge study
to make sure that the infection system works and to act
as a comparison.
Volunteers are exposed to the bites of five malaria-infected
mosquitoes. These mosquitoes are placed in paper cups onto
which the volunteers rest their arm for five minutes.
|
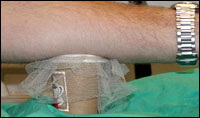 A volunteer in the challenge study
A volunteer in the challenge study |
 mosquitoes carrying the malaria
parasite
mosquitoes carrying the malaria
parasite |
Clearly, untreated malaria could be very serious
and so the volunteers are followed-up very carefully in our clinic
(up to twice per day). At each of these visits a blood sample
is taken and examined for malaria. At the first sign of malaria,
the volunteer is immediately treated with the anti-malarial drug
chloroquine. However, if the volunteer is completely protected
by the vaccine they will not develop malaria.
|
|
|
|
A malaria blood film
Image from the Wellcome Trust
courtedy of Bruce-Chwatt LJ
|
Microscopic appearance
of malaria infection
Image from Liverpool School
of Tropical Medicine
|
The aim of the malaria challenge study is to establish whether
our vaccination regimes offer protection against malaria infection.
Protection may be 'partial' or 'complete':
Complete protection is where our vaccinated
volunteers do not develop malaria in the challenge study. Naturally,
all the unvaccinated control volunteers have to develop malaria
so that we are confident that the infection system has worked.
Partial protection is where there is a delay
in the onset of malaria in the vaccinated volunteers compared
to the unvaccinated controls. This means that the body's immune
system is controlling the infection to start with but is ultimately
overwhelmed. Partial protection seen in the UK may turn into a
more significant degree of protection in Africans who have already
been exposed to malaria.
Using different combinations of our malaria vaccines we have been
able to completely protect some volunteers against malaria infection.
Other volunteers have been partially protected against malaria.
This is clearly very exciting progress although there is more
work to do and larger field studies to be planned.
|





